Background
Reversible cerebral vasoconstriction syndrome (RCVS) is a rare complication of packed red blood cell (pRBC) transfusion primarily reported in adults with severe chronic anemia due to menorrhagia. It is characterized by a triad of rapidly changing and reversible segmental cerebral artery narrowing and dilatation, thunderclap headache, and benign cerebrospinal fluid findings. 1,2 More than 50% of patients experience blurring of vision or cortical vision loss, and a subset of patients develop hemiparesis or aphasia. 1 There are emerging case reports of RCVS in pediatric patients, however the literature examining this transfusion complication in children is limited. The rarity and potential severity of RCVS warrants better understanding by pediatric hematology and transfusion providers.
Results
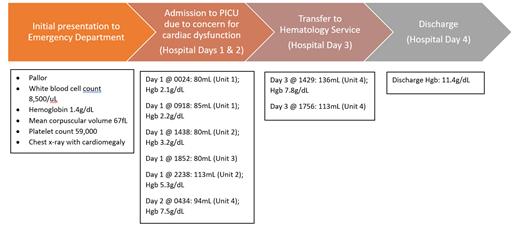
We describe a previously healthy 4 year-old male who presented with pallor and laboratory parameters consistent with severe iron deficiency anemia. Chest x-ray showed cardiomegaly, and echocardiogram demonstrated pericardial effusion, moderate left ventricular dilation, and moderate coronary artery dilatation. His history was notable for an extremely selective diet and at least 50 ounces of cow's milk daily. The patient was admitted, and over the course of 3 days, he received a total of 43 mL/kg of pRBCs (602 mL total) in 8 aliquots, and his hemoglobin (Hgb) increased from 1.4 g/dL to 11.4 g/dL (Fig. 1).
He then re-presented 5 days after discharge with altered mental status and malignant hypertension. Brain MRI/MRA was consistent with RCVS, with luminal irregularities in multiple anterior and posterior circulation intracranial arteries creating a beaded appearance. He was treated supportively with aggressive blood pressure control and made a complete neurologic recovery 8 days after presentation. At hematology clinic follow-up 2 months post-discharge, he remained at his typical neurologic baseline.
Conclusions
The connection between blood transfusion and RCVS is postulated to be due to a rapid increase in hematocrit and blood viscosity, which alters cerebral autoregulation and leads to hypertensive encephalopathy despite systemic normotension. 3 The increased viscosity may also lead to vasospasm. 3 When a patient with severe chronic anemia receives a pRBC transfusion, there is increased cerebral blood flow and viscosity, resulting in decreased vasodilation, increased vascular resistance, and cerebral vessel constriction. However, these are hypothesized mechanisms with little supportive experimental data. Vasculopathies and vasoconstrictive medications 2 are also risk factors for RCVS.
There are 4 previous reported cases of pediatric RCVS associated with pRBC transfusion. 2 patients were acutely ill with complications of sickle cell anemia. 7,9 Another case occurred in a patient with systemic lupus erythematosus 8, and the fourth occurred in the setting of severe iron deficiency anemia. 5 Several features of our patient's clinical course are similar to the reported cases of transfusion-related RCVS 5 and warrant highlighting: 1) severe chronic anemia with cardiac dysfunction and 2) the large volume of transfused pRBCs over a short period resulting in a significant increase in Hgb. However, our patient developed symptoms of RCVS sooner than other published cases and did not have seizure or stroke.
Pediatric guidelines recommend pRBC transfusion for Hgb levels between 5-10 g/dL, depending on the clinical scenario. 4 There are few data to guide Hgb targets for pediatric patients presenting with severe anemia. Over-transfusion does not offer physiological benefit and poses risk of transfusion-related adverse events. A case report from Dou et al 6 proposed that a >5 g/dL Hgb increase was associated with a greater likelihood of developing RCVS. For this patient with cardiac dysfunction and social concerns including lack of primary care, the potential benefits of achieving a higher Hgb with pRBC transfusions were prioritized.
RCVS should be considered in pediatric and adult patients recently transfused for chronic severe anemia presenting with headaches or other neurological symptoms. Prompt recognition of RCVS may prevent unnecessary treatment and potentially limit risk of neurologic compromise. The role of the volume and rapidity of the pRBC transfusions in the development of RCVS warrants further study.
Fig 1. Timeline of initial presentation, detailing blood transfusions
Disclosures
Samelson-Jones:Genentech: Consultancy; Pfizer: Consultancy, Honoraria; Biomarin: Consultancy; Amarna: Current holder of stock options in a privately-held company; GeneVentiv: Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal